ResMed
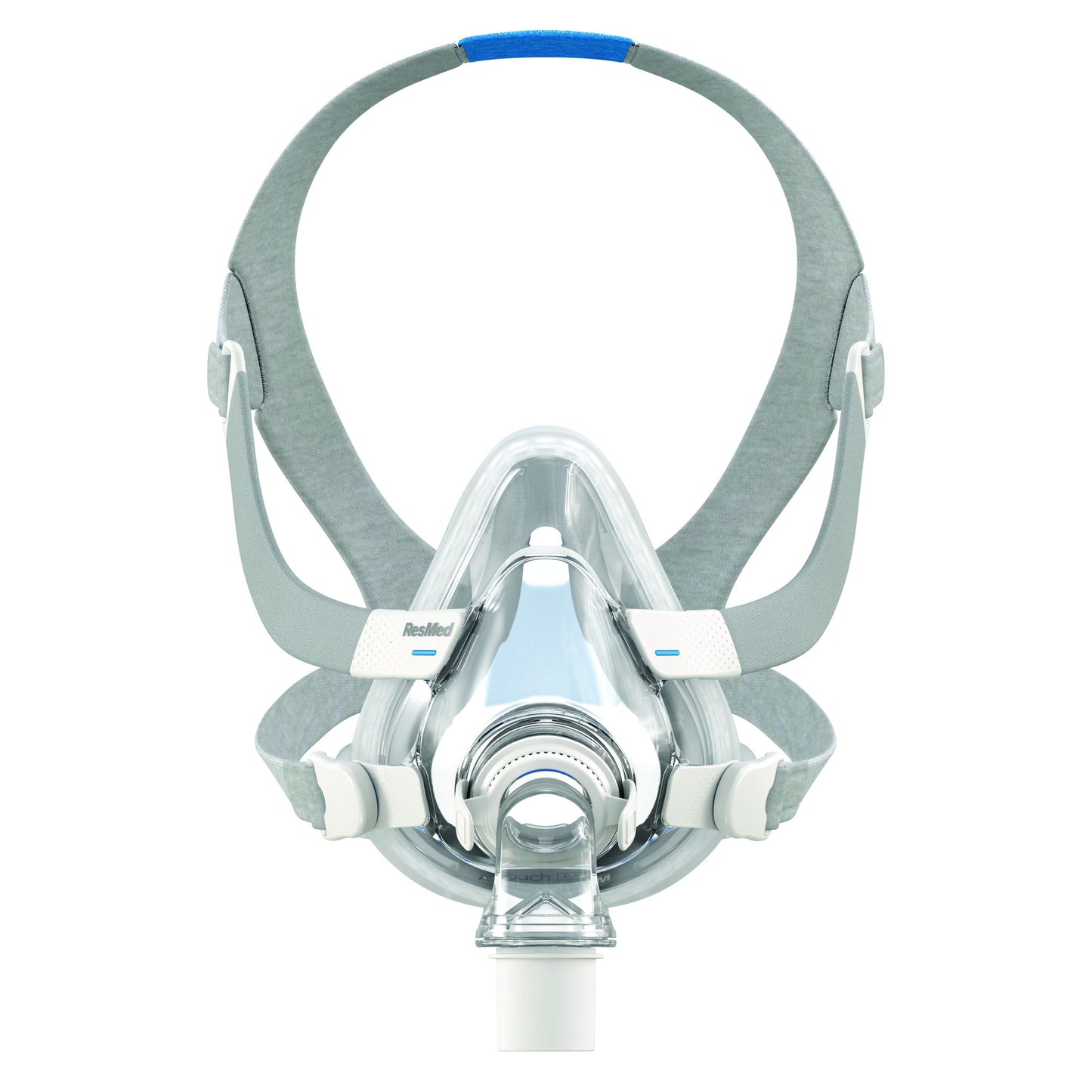
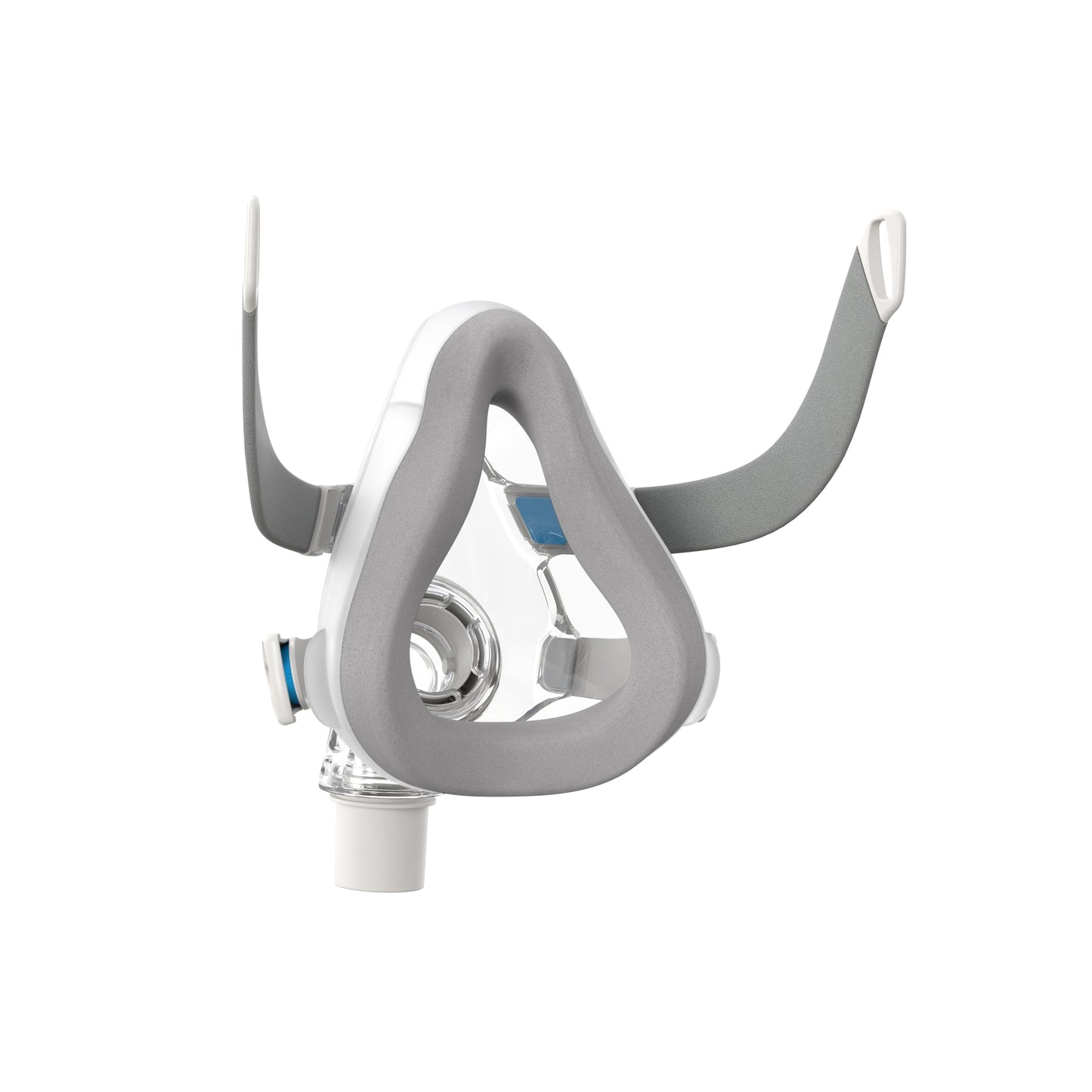
AirTouch F20 full face mask
AirTouch F20 full face mask
Couldn't load pickup availability
The AirTouch F20 Full Face Mask is ultra soft thanks to its memory foam cushion. You can count on its reliable sealing for a whole range of therapeutic pressures. Silent, its noise is reduced to a minimum thanks to its ventilation system.
Important info: this mask is not recommended if you have a pacemaker , defibrillator or other active medical implant.*
The manufacturer recommends replacing the cushion after 1 to 2 months of use or at the first signs of deterioration.
Main characteristics
Comfortable
- Adapts to the unique contours of every face with its UltraSoft memory foam sealing cushion.
- Light and breathable, its cushion stays in place all night and does not leave red marks on the face.
- Suitable for everyone, regardless of nose shape or facial hair.
Waterproof
- The cushion provides a reliable seal all night long, especially at high pressure.
Silent
- The QuietAir ventilation system gently diffuses the exhaled air, so that noise is kept to a minimum.
Easy to use and clean
- Its quick-release connection makes it easy to disconnect tubing without having to remove the mask.
- A quick wipe with an alcohol-free wipe will keep the cushion clean.
- Installs quickly and easily with magnetic clips.
Practical
- Compatible with the AirMini travel device.
Easy to maintain
• Cushion requires no cleaning.
• The nasal cushion should be replaced if it shows signs of deterioration, usually after one or two months of use.
* Manufacturer's recommendation : Magnets are used in the lower harness straps and in the rigid frame of the AirFit N20. Ensure that the headgear and frame are kept a minimum distance of 50 mm (2 inches) from any active medical implant (eg pacemaker or defibrillator) to avoid possible effects of localized magnetic fields. The strength of the magnetic field is less than 400 mT.
Contraindication
The use of masks with magnetic components is contraindicated in patients with the following pathologies or pre-existing conditions:
- Metallic hemostatic device implanted in the skull following an aneurysm;
- Metallic flakes in one or both eyes.
Share